


Next: 8.2.2 Molecular oxygen
Up: 8.2 Spectroscopy of HO, O, O
Previous: 8.2 Spectroscopy of HO, O, O
The rotational energy level diagram of water is shown on Fig.8.2. Each
level is denoted, as usual for asymmetric top molecules, by three numbers
JK-1,K+1. J, which is a ``good'' quantum number, represents the total
angular momentum of the molecule; by analogy with symmetric tops, K-1 and
K+1 stand for the rotational angular momenta around the axis of least and
greatest inertia. Allowed radiative transitions obey the selection rules
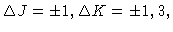 with
with
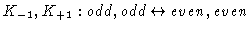 or
or
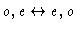 . The levels with K-1 and K+1 of the
same parity are called para levels, those of opposite parity, ortho
levels. Transitions between ortho and para levels are forbidden; due to the presence
of two symmetrical hydrogen nuclei, the ortho levels have a nuclear statistical
weight 3 times larger than the para levels of same J (see e.g. [Townes & Schawlow 1975]).
The arrows in Fig.8.2 show the transitions which will be observed by the
satellite FIRST (J.Cernicharo, private communication).
. The levels with K-1 and K+1 of the
same parity are called para levels, those of opposite parity, ortho
levels. Transitions between ortho and para levels are forbidden; due to the presence
of two symmetrical hydrogen nuclei, the ortho levels have a nuclear statistical
weight 3 times larger than the para levels of same J (see e.g. [Townes & Schawlow 1975]).
The arrows in Fig.8.2 show the transitions which will be observed by the
satellite FIRST (J.Cernicharo, private communication).
Figure:
The
rotational energy level diagram of water vapor
![\resizebox{12.0cm}{!}{\includegraphics[angle=270]{mg1f2.eps}}](img700.gif) |



Next: 8.2.2 Molecular oxygen
Up: 8.2 Spectroscopy of HO, O, O
Previous: 8.2 Spectroscopy of HO, O, O
S.Guilloteau
2000-01-19
![\resizebox{12.0cm}{!}{\includegraphics[angle=270]{mg1f2.eps}}](img700.gif)